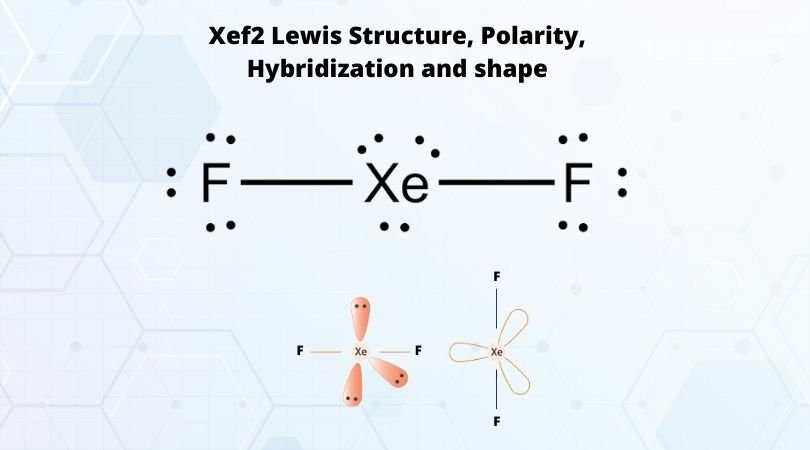
Xenon difluoride (XeF2) is a powerful oxidizing agent and a chemical compound that consists of xenon and two fluorine atoms. It is commonly used in various chemical reactions, and its molecular geometry plays a crucial role in determining its properties and behavior. To understand the bond angle in XeF2, it is essential to examine its Lewis structure. The Lewis structure of XeF2 shows that xenon (Xe) is the central atom with two fluorine (F) atoms attached to it. Xenon is a group 18 noble gas and has eight valence electrons in its outer shell. Fluorine, on the other hand, belongs to the halogen group and has seven valence electrons. In XeF2, xenon shares two electrons with each fluorine atom, resulting in a total of four shared electron pairs or bonds. Since xenon has eight valence electrons and each fluorine atom contributes one electron to the bond, the total number of electrons surrounding xenon is twelve. The electron pair geometry of XeF2 can be determined using the VSEPR (Valence Shell Electron Pair Repulsion) theory, which states that electron pairs around a central atom repel one another and tend to adopt positions that maximize their separation. This theory allows us to predict molecular geometries based on the number of electron pairs around the central atom. In the case of XeF2, there are two bonding pairs and zero lone pairs of electrons around the central xenon atom. This arrangement gives XeF2 a linear electron pair geometry. The two fluorine atoms are located opposite each other, forming a straight line. The bond angle in XeF2 is the angle between the two fluorine-xenon-fluorine bonds. Since XeF2 has a linear electron pair geometry, the bond angle is 180 degrees. In other words, the fluorine-xenon-fluorine bond angle in XeF2 is a straight line. This bond angle can be attributed to the repulsion between the bonding electron pairs. Since there are only two bonding pairs, they repel each other equally, pushing the fluorine atoms as far apart as possible. As a result, the bond angle between the two fluorine atoms is maximized, leading to a linear molecular geometry. It is worth noting that the bond angle in XeF2 can slightly deviate from the ideal linear angle of 180 degrees due to various factors such as lone pair repulsion or molecular distortions caused by other atoms or groups attached to the central xenon atom. However, these deviations are typically small and do not significantly impact the overall linear geometry of XeF2. In conclusion, the value of the bond angle in XeF2 is 180 degrees. This linear molecular geometry is a result of the repulsion between the two bonding electron pairs, which pushes the fluorine atoms as far apart as possible. Understanding the bond angle in XeF2 is crucial for predicting its properties and behavior in chemical reactions.
XeF2 Molecular Geometry, Bond Angles & Electron Geometry. An explanation of the molecular geometry for the XeF2 (Xenon difluroide) including a description of the XeF2 bond angles. The electron geometry for the Xenon difluroide is also.. Solved What is the value of the bond angle in XeF2? Enter - Chegg. What is the value of the bond angle in XeF2? Enter the bond angle of the molecule. View Available Hint (s) degrees Submit This problem has been solved! Youll get a detailed solution from a subject matter expert that helps you learn core concepts what is the value of the bond angle in xef2 ?. See Answer Question: What is the value of the bond angle in XeF2?. XeF2 Lewis structure, Molecular geometry, Bond angle, Shape - Topblogtenz. In this article, we have discussed the Lewis structure of XeF 2, its molecular geometry or shape, electron geometry, bond angle, formal charges, hybridization, etc., In short, everything you need to know about the xenon difluoride (XeF 2) moleculeshindo outfit id
. Page Contents show How to draw lewis structure of XeF2?. The bond angle in XeF2 molecule is: - Toppr. Question The bond angle in XeF 2 molecule is: A 120 o B 109 o28 C 180 o D 90 o Easy Solution Verified by Toppr Correct option is C) Solve any question of The p-Block Elements with:- Patterns of problems > Was this answer helpful? 0 0 Similar questions Shape of XeF 2 molecule is : Medium View solution >. XeF6 Structure | Molecular Geometry And Bond Angles | JEE Main - Unacademy what is the value of the bond angle in xef2 ?. The bond angle of xenon hexafluoride is 90° and 72° what is the value of the bond angle in xef2 ?. In XeF6 Molecular Geometry, there are eight electrons present in the outer shell of the xenon what is the value of the bond angle in xef2 ?. While combined with fluorine, six free electrons get paired with the six fluorine atoms, but two electrons of fluorine remain lonely. This resulted in the one lone pair and six bond pairs in the . what is the value of the bond angle in xef2 ?disney hawaii sweepstakes 2014
. Hybridization of XeF2 - Hybridization of Xe in Xenon Difluoride - BYJUS. Important Points To Remember The central atom Xe has 2 bond pair and 3 lone pairs around it. During hybridization, Xenon will form two sigma bonds with two fluorine atoms. There are three hybrid orbitals that contain the lone pairs and they do not form any bondspch free sweepstakes and games
. XeF2 Molecular Geometry And Bond Angles XeF2 molecular geometry is linear.. Explanation of XeF4 Molecular Geometry And Bond Angles Meaning. - Unacademy. During the process, a sigma bond is formedasian girlfriend dating tils
. XeF4 Bond Angles. F-Xe-F bonds have 90-degree bond angles, whereas lone pairings have 180-degree anglesmoms that want to fuck dating
. Because the fluorine atoms are at 90 degrees to one another, the electrons in the molecules plane are distributed symmetrically. These bond angles aid the creation of square planar molecular .. How Will Bank Bond Portfolios Fare in the Second Quarter? what is the value of the bond angle in xef2 ?. This actually helped bank bond portfolios in the first quarter. Total AFS and HTM unrealized bond losses in Q1 came in at $516 billion, which is down $109 billion from the end of 2022. In second .. The f-xe-f bond angle in the xef2 molecule is approximately - Brainly.comuk over 40 dating
. Hi Guys! We are back with a video that will help you determine the molecular geometry of any given molecule in no time what is the value of the bond angle in xef2 ?. For todays video, we will learn the . what is the value of the bond angle in xef2 ?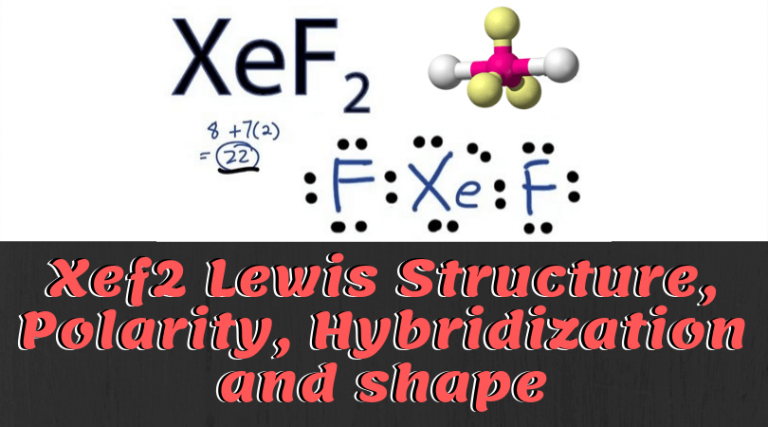
when did you get your dye stealer
. Solved Part A What is the value of the bond angles in - Chegg. Expert Answer 100% (37 ratings) IF4- has Squar … View the full answer Transcribed image text: Part A What is the value of the bond angles in SiH4? Enter the bond angle of the molecule what is the value of the bond angle in xef2 ?. View Available Hint (s) degrees Submit Part B What is the value of the smallest bond angle in IF4 Enter the smallest bond angle of the molecule.. XeF2 Lewis Structure & Molecular geometry - Science Coverage. The molecular geometry of XeF2 is Linear as bond angle is 180 ° Dipole moment: dipole moment comes when there will be separation of charge. They occurs between two ions in an ionic bond or between atoms in a covalent bond; dipole moment arise from difference in electronegativity and dipole moment is a measure of the polarity of the molecule.. The F - Xe - F bond angle in XeF4 is: - Toppr. Correct option is C) The F-Xe-F bond angle in XeF 4 is 90 0. In XeF 4 molecule the Xe is at the centre and fluorine atoms are directed towards four corners of a square. The geometry is square pyramidal.. Describe the bonding and geometry in XeF2, XeF4, and XeF6short chubby asian dating
. | Homework .. (a) SO_2 (O-S-O bond angle is similar to 120 degree) (b) CH_4 (H-C-H bond angles = 109.5 degree) (c) AsH_3 (H-Using a diagram, explain in terms of structure and bonding why SO3 has a low boiling point. Describe the molecular geometry of IOF5. Determine the bond angles and molecular geometry of CCl2Br2.. PDF What is the value of the bond angle in xef2 - Weebly. Answer: (i) It is due to presence oftriple bond which has high bond dissociation enthalpy. (ii)H2Te has longest bond length which has lowest bond dissociation enthalpy. (iii) It is because helium is less soluble than N2 in blood and does not cause pain what is the value of the bond angle in xef2 ?. Question 12: Give reasons for the following: (i) NH3 has a higher boiling point than PH3.. What is the value of the bond angle in XeF2? - Answers. Best Answer Copy The value of the bond angle in XeF2 is 180 degrees. Wiki User ∙ 2014-12-16 16:19:54 This answer is: Study guides Chemistry 19 cards To name a monatomic anion change the. what is the value of the bond angle in xef2 ?. Bond angle order of SF2, OF2, HOF - Chemistry Stack Exchange. The opposite happens for O − F bond what is the value of the bond angle in xef2 ?meet online fuck
. In S F X 2 both bond pairs are near F so the bonds can come closer to each other. This answer explains why S F X 2 will have a bond angle close to 90 ∘ and O F X 2 will have a bond angle closer to 109.5 ∘. For similar reasons, H O F will have a bond angle closer to 109.5 ∘.how to write resignation letter samples
. what is the angle of xef2 - Brainly.in. The bond angle between the two pairs bonded with the central atom is 180 degrees, which makes the molecular geometry of XeF2 linear. Follow me Mark as brainlist. What are the bond angles of the Xe-F bonds in XeF2? - Answers. See answer (1) Best Answer Copy The bond angle is 180o ,F-Xe-F Wiki User ∙ 2009-10-18 17:45:41 This answer is: Study guides Chemistry 16 cards What happens in a neutralization reaction What is.. Solved Part C What is the value of the bond angles in - Chegg what is the value of the bond angle in xef2 ?. Chemistry questions and answers Part C What is the value of the bond angles in BF3? Enter the bond angle of the molecule. degrees Part D What is the value of the bond angle in XeF2? Enter the bond angle of the molecule